Background:
Tissue regeneration is the process of replacing, repairing, or regrowing damaged or lost tissues with the development of a biological substitute known as a scaffold or micro-implant, which allows the delivery of cells to the target tissue. It offers the potential to revolutionise medicine by healing damaged organs and tissues. This could address a range of conditions, from chronic wounds to organ failure, such as nerve or eye. However, despite significant advancements, current methods still face limitations. Designing scaffolds that accurately mimic the complex structures of natural tissues and optimising cell growth within these scaffolds remain significant challenges.
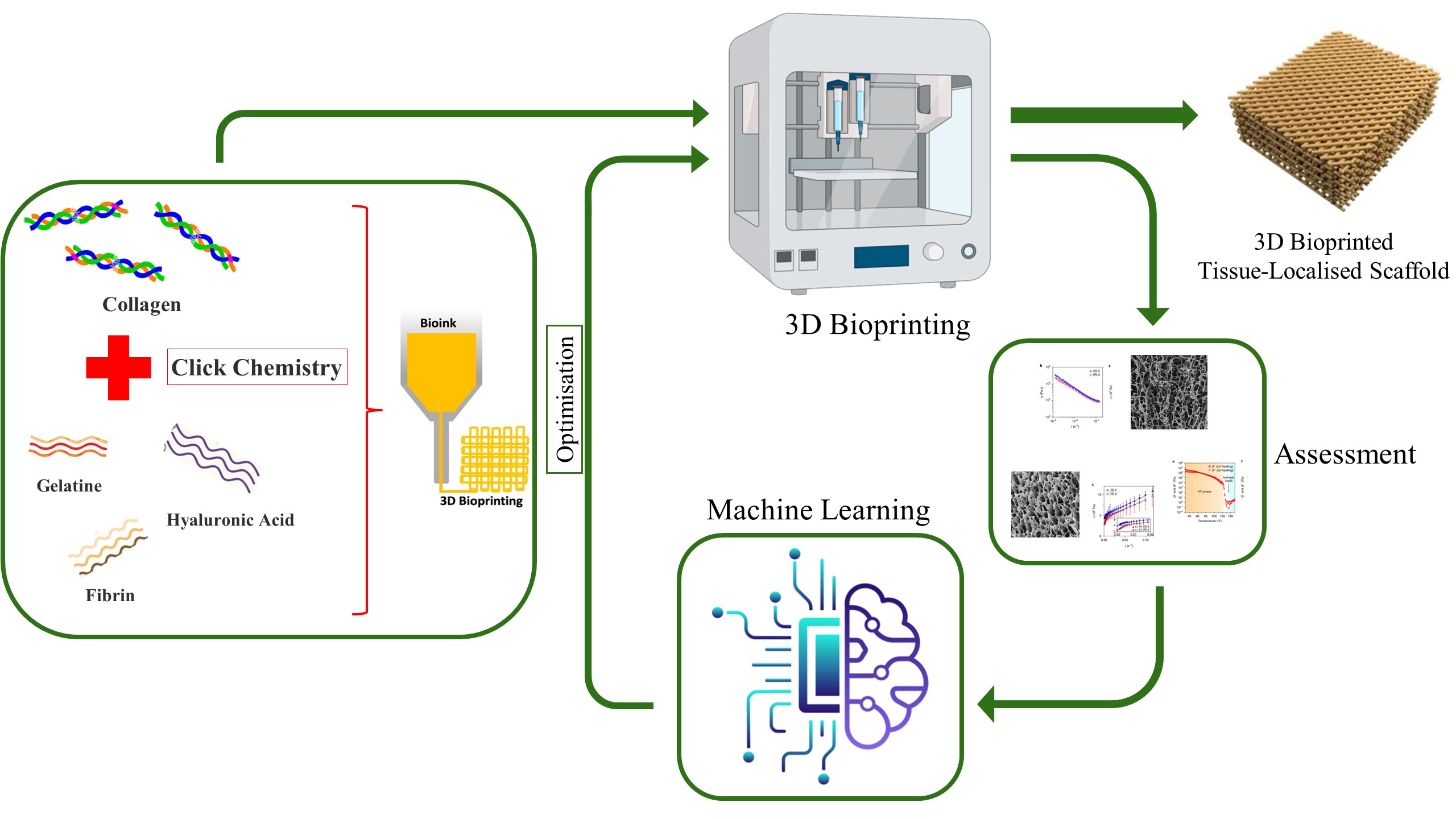
3D bioprinting has emerged as a powerful tool in tissue engineering, allowing for the creation of intricate scaffolds that mimic natural tissues. These scaffolds provide a crucial support structure for cells, promoting their growth and organisation into functional tissues. Among various approaches to printing, extrusion-based 3D bioprinting has been widely used due to its cost-effective operation and ability to be used for a wide range of biomaterials. However, this pioneering technology demands optimised parameters for obtaining good printability and cell viability. Optimising scaffold printability requires a thorough understanding of the biomaterial's biological, mechanical, and rheological properties. Illustrating complex relationships in a multiparameter process is not straightforward through conventional methods. It can be overcome with the implementation of artificial intelligence (AI). Machine learning techniques have the potential to revolutionise this field. By analysing vast datasets on biomaterials, cell behavior, and tissue structure, machine learning can guide the design of superior scaffolds with properties tailored to specific organ needs. By optimising cell placement or cell pattern within the scaffolds, AI fosters the development of tissues that are not only more functional but also better organised, mimicking natural structures. This enhanced precision and efficiency pave the way for advanced therapeutic applications in 3D bioprinting.
This PhD proposal aims to use 3D bioprinting to fabricate a 3D tissue localised scaffold with a specific shape and size containing cells to replace damaged cells. Identifying suitable printing conditions for scaffold requires extensive experimentation in a time and resource-demanding process, hence we aim to use machine learning methods to predict some of the printing configurations.
Aims:
The primary aim of this research project is to develop and validate an AI-driven framework for the optimised design and fabrication of collagen-based micro-implants for tissue regeneration applications.
Methods:
The proposed project will use a combination of experimental and computational methods. The experimental work will involve the fabrication of a variety of tissue engineering scaffolds using different materials, printing parameters, and fabrication techniques. The computational work will involve the development of AI algorithms for the prediction of scaffold properties and the optimization of scaffold design. The specific methods that will be used include:
- 3D bioprinting to fabricate tissue engineering scaffolds.
- Quality analysis and assessments to characterize the structure of the tissue.
- Mechanical testing to measure the mechanical properties of scaffolds.
- Machine learning to predict the properties of scaffolds and optimize their design.
Significance:
The development of AI-driven methods for the design of tissue engineering scaffolds has the potential to revolutionize the field of tissue engineering. These methods will make it possible to design scaffolds that are more efficient, effective, and affordable. The methods will also make it possible to design scaffolds that are tailored to specific applications, such as the repair or regeneration of damaged tissues such as retina or nerve cells.
For more information about the project, please contact Professor Massoud Zolgharni